Isoenzymes are proteins with a different structure that catalyze the same reaction. Frequently they are oligomers made with different polypeptide chains, so they usually differ in regulatory mechanisms and in kinetic characteristics.
From the physiological point of view, isozymes allow the existence of similar enzymes with different characteristics, “customized” to specific tissue requirements or metabolic conditions.

One example of the advantages of having isoenzymes for adjusting the metabolism to different conditions and/or in different organs is the following: Glucokinase and Hexokinase are typical examples of isoenzymes.
In fact, there are four Hexokinases: I, II, III and IV. Hexokinase I is present in all mammalian tissues, and Hexokinase IV and Glucokinase is found mainly in the liver, pancreas, and brain.
Both enzymes catalyze the phosphorylation of Glucose:
Glucose + ATP —–> Glucose 6 (P) + ADP
Hexokinase I has a low Km and is inhibited by glucose 6 (P). Glucokinase is not inhibited by Glucose 6 (P) and his Km is high.
These two facts indicate that the activity of glucokinase depends on the availability of the substrate and not on the demand of the product.
Since Glucokinase is not inhibited by glucose 6 phosphate, in conditions of high concentrations of glucose this enzyme continues phosphorylating glucose, which can be used for glycogen synthesis in the liver.
Additionally, since Glucokinase has a high Km, its activity does not compromise the supply of glucose to other organs; in other words, if Glucokinase had a low Km, and since it is not inhibited by its product.,
It would continue converting glucose to glucose 6 phosphate in the liver, making glucose unavailable for other organs (remember that after meals, glucose arrives first to the liver through the portal system).
Since isoenzymes have different tissue distributions, their study is an important tool in assessing the damage to specific organs.
Examples of the diagnostic use of isoenzymes are the study of Lactate Dehydrogenase and Creatine Kinase.
Table of Contents
IsoEnzymes 1
Lactate Dehydrogenase (LDH)
It is formed by the association of five peptide chains of two different kinds of monomers: M and H.
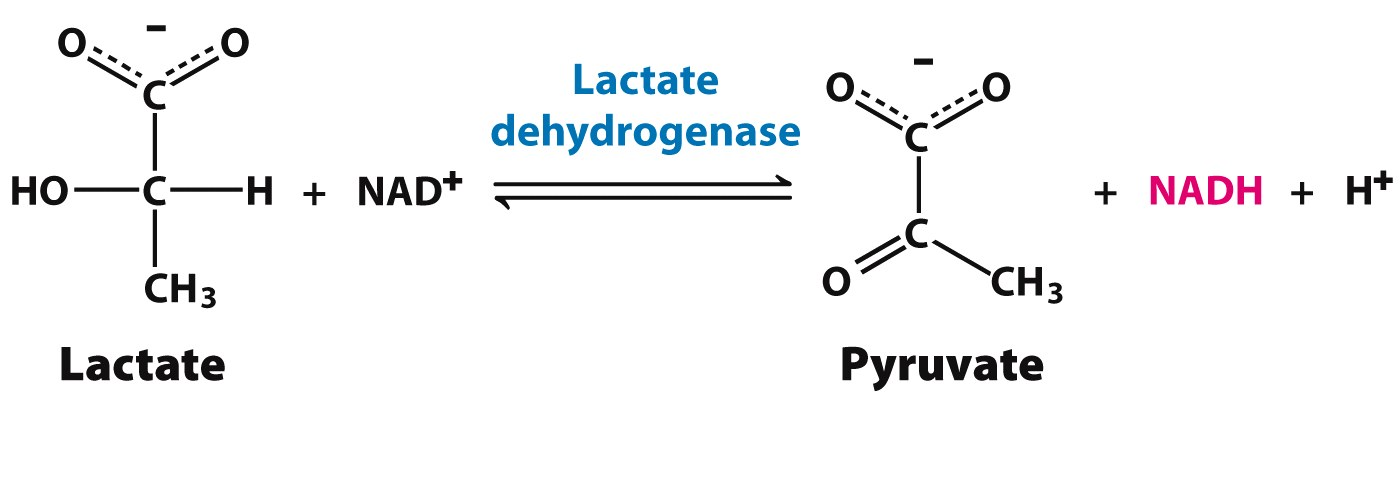
The variants seen in humans are:
- LDH1: M M M M (abundant in heart, brain erythrocytes; around 33% of serum LDH)
- LDH2: M M M H (abundant in heart, brain erythrocytes; around 45% of serum LDH)
- LDH3: M M H H (abundant in the brain, kidneys, lung; around 18 % of serum LDH)
- LDH4: M H H H ((abundant in liver, skeletal muscle, kidney; around 3% of serum LDH)
- LDH5: H H H H ((abundant in liver, skeletal muscle, ileum; around 1 % of serum LDH)
In myocardial infarction, Total lactate dehydrogenase (LDH) increases, and since heart muscle contains more LDH1 than LDH2, LDH1 becomes greater than LDH2 between 12 and 24 hours.
After the infarction, so the ratio LDH1/LDH2 becomes higher than 1 and will stay flipped for several days.
An increase of LDH 5 in serum is seen in different hepatic pathologies: cirrhosis, hepatitis, and others. An increase of LDH5 in heart diseases usually indicates secondary congestive liver involvement.
Isoenzyme 2 – Creatine Kinase
Creatine Kinase (CK) and Creatine phosphokinase (CPK) is a similar example: three isoenzymes formed by combinations of different subunits:
- CK1 (BB) is abundant in thebrain and smooth muscle (practically absent from serum)
- CK2 (MB) is abundant in cardiac muscle, some in skeletal muscle (practically absent from serum)
- CK3 (MM) is abundant in skeletal muscle and cardiac muscle (practically 100 % of serum CK)
They can be differentiated based on their different electrophoretic mobility.
The primary clinical use of CK studies is the diagnosis of Myocardial Infarction, (increased in the MB variant), but CK is also increased in different conditions as muscular diseases and traumas (MM and MB) and brain trauma and brain surgery (BB).
CK2 appears in serum within 6 hours after the myocardial infarction and is cleared after 24 to 48 hours.
A persistence of CK2 in serum indicates the extension of the infarction to other areas or another infarction.
Isoenzymes and allozymes as molecular markers
Population genetics is essentially a study of the causes and effects of genetic variation within and between populations, and in the past, isoenzymes have been amongst the most widely used molecular markers for this purpose.
Although they have now been largely superseded by more informative DNA-based approaches (such as direct DNA sequencing, single nucleotide polymorphisms, and microsatellites).
They are still amongst the quickest and cheapest marker systems to develop and remain (as of 2005) an excellent choice for projects that only need to identify low levels of genetic variation, e.g. quantifying mating systems.
Source: Iso-enzymes
Check the Knowledge with this MCQ by clicking this “Start” Button
[mtouchquiz 3]