What is an enzyme? Enzymes are organic molecules, pretentious in nature that are specialized to catalyze biological reactions. Here these can be termed as “Biological Catalysts” (or) “Biocatalysts” (or) “Middle man of Nature”.
Table of Contents
What is an Enzyme? What is the meaning?
The name ‘enzyme‘ (enG=in; zymeG=Yeast) literal means “in yeast“. This was referred to denote one of the most noteworthy reactions wherein the production of ethyl alcohol and carbon dioxide through the agency of an enzyme, the zymase, present in yeast takes place. This reaction is most popularly known as “Alcoholic fermentation”.
Today nearly 2000 different enzymes are known and 200 enzymes have been crystallized from catalytic power and specificity are the most sticking characteristics of enzymes. The activity of most of the enzymes is regulated. Enzymes accelerated biochemical reactions by factors of a million or more. In fact, in the absence of enzymes, most of the reactions in biological systems do not occur at perceptible rates.
As an example, the catalytic activity of carbonic anhydrase can be cited; hydration of carbon dioxide (CO2) into carbonic acid is brought about y carbonic anhydrase, this is one of the fastest enzymes. Each enzyme molecule can hydrate 105 molecules of CO2 per second. The same reaction without the enzyme will be 107 times slower than the catalytic reaction.

What is Enzyme Catalysis?
A catalyst is a chemical that increases the rate of a chemical reaction without itself being changed by the reaction. The fact that they aren’t changed by participating in a reaction distinguishes catalysts from substrates, which are the reactants on which catalysts work.
Enzymes catalyze biochemical reactions. They are similar to other chemical catalysts in many ways:
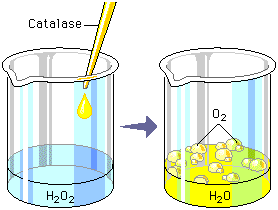
- Enzymes and chemical catalysts both affect the rate but not the equilibrium constant of a chemical reaction. Reactions proceed downhill energetically, in accord with the Second Law of Thermodynamics. Catalysts merely reduce the time that a thermodynamically favored reaction requires to reach equilibrium.
- Remember that the Second Law of Thermodynamics tells whether a reaction can occur but not how fast it occurs.
- Enzymes and chemical catalysts increase the rate of a chemical reaction in both directions, forward and reverse. This principle of catalysis follows from the fact that catalysts can’t change the equilibrium of a reaction. Because a reaction at equilibrium occurs at the same rate both directions, a catalyst that speeds up the forward but not the reverse reaction necessarily alters the equilibrium of the reaction.
- Enzymes and chemical catalysts bind their substrates, not permanently, but transiently—for a brief time. There is no action at a distance involved. The portion of an enzyme that binds substrate and carries out the actual catalysis is termed the active site.
Enzymes differ from ordinary chemical catalysts in several important respects
- Enzymes are specific: Chemical catalysts can react with a variety of substrates. For example, hydroxide ions can catalyze the formation of double bonds and also the hydrolysis of esters. Usually, enzymes catalyze only a single type of reaction, and often they work only on one or a few substrate compounds.
- Enzymes work under mild conditions: Chemical catalysts often require high temperature and/or pressure to function. For example, nitrogen can be reduced to ammonia industrially by the Haber process, catalyzed by iron at 500° C. and at 300 atmospheres pressure of N2. In contrast, the same reaction is carried out enzymatically at 25°C. and less than 1-atmosphere pressure of N2. These gentle conditions of temperature, pressure, and pH characterize enzymatic catalysis, especially within cells.
- Enzymes are stereospecific: Chemical catalysis of a reaction usually leads to a mixture of stereoisomers. For example, the addition of acid-catalyzed water to a double bond leads to an equimolar (50:50) mixture of D and L isomers where the water is added. In contrast, catalysis of water addition by enzymes results in the complete formation of either the D or L isomer, but not both.
- Enzymes are macromolecules: The macromolecules are composed of protein, or in a few cases, RNA. Most chemical catalysts are either surfaces, for example, metals like platinum, or else small ions, such as hydroxide ions.
- Enzymes are often regulated: The regulation occurs either by the concentration of substrates, by binding small molecules or other proteins, or by covalent modification of the enzymes’ amino acid side chains. Thus, an enzyme’s effectiveness can be altered without changing the concentration of the enzyme; on the other hand, the effectiveness of a chemical catalyst is generally determined by its overall concentration.
Six types of Enzyme Catalysis
Although a huge number of reactions occur in living systems, these reactions fall into six types of enzyme catalysis reactions. The reactions are:
1. Oxidation and reduction: This is the frequently abundant enzyme catalysis reactions in metabolism. Enzymes that carry out these reactions are called oxidoreductases. For example, alcohol dehydrogenase converts primary alcohols to aldehydes. In the given reaction, ethanol is converted to acetaldehyde, and the cofactor, NAD, is converted to NADH. In other words, ethanol is oxidized, and NAD is reduced. (The charges don’t balance, because NAD has some other charged groups.) Remember that in redox reactions, one substrate is oxidized and one is reduced.
![]()
2. Group transfer reactions: These enzymes, called transferases, move functional groups from one molecule to another. For example, alanine aminotransferase shuffles the alpha-amino group between alanine and aspartate: Other transferases move phosphate groups between ATP and other compounds, sugar residues to form disaccharides, and so on.
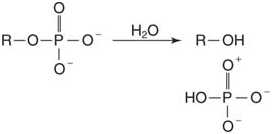
3. Hydrolysis: These enzymes, termed hydrolases, break single bonds by adding the elements of water. For example, phosphatases break the oxygen-phosphorus bond of phosphate esters:
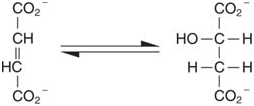
4.Formation or removal of a Double bond with group transfer: The functional groups transferred by these lyase enzymes include amino groups, water, and ammonia. For example, decarboxylases remove CO2 from alpha- or beta-keto acids: Dehydratases remove water, as in fumarase (fumarate hydratase):
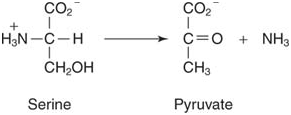 5.Isomerization of functional groups: In many biochemical reactions, the position of a functional group is changed within a molecule, but the molecule itself contains the same number and kind of atoms that it did in the beginning. In other words, the substrate and product of the reaction are isomers. The isomerases (for example, triose phosphate isomerase, shown following), carry out these rearrangements.6. Single bond formation by eliminating the elements of water: Hydrolases break bonds by adding the elements of water; ligases carry out the converse reaction, removing the elements of water from two functional groups to form a single bond. Synthetases are a subclass of ligases that use the hydrolysis of ATP to drive this formation. For example, aminoacyl-transfer RNA synthetases join amino acids to their respective transfer RNAs in preparation for protein synthesis; the action of glycyl-tRNA synthetase is illustrated in this figure: Deaminases remove ammonia, for example, in the removal of amino groups from amino acids:
5.Isomerization of functional groups: In many biochemical reactions, the position of a functional group is changed within a molecule, but the molecule itself contains the same number and kind of atoms that it did in the beginning. In other words, the substrate and product of the reaction are isomers. The isomerases (for example, triose phosphate isomerase, shown following), carry out these rearrangements.6. Single bond formation by eliminating the elements of water: Hydrolases break bonds by adding the elements of water; ligases carry out the converse reaction, removing the elements of water from two functional groups to form a single bond. Synthetases are a subclass of ligases that use the hydrolysis of ATP to drive this formation. For example, aminoacyl-transfer RNA synthetases join amino acids to their respective transfer RNAs in preparation for protein synthesis; the action of glycyl-tRNA synthetase is illustrated in this figure: Deaminases remove ammonia, for example, in the removal of amino groups from amino acids:
Enzyme catalysis is an important aspect in enzymology and in all biological science areas.
Please share these notes with your friends, who are preparing for examinations in biology related. Just use below social icons, sharing process will be easy. Thanks for sharing.