Lipids are a diverse group of biomolecules that play crucial roles in various biological processes. These organic compounds are essential for life, serving as energy storage molecules, structural components of cell membranes, and signaling molecules in cellular communication. The study of lipids, known as lipidology, has gained significant attention in recent years due to its implications for human health, disease, and technological applications.
This article provides a comprehensive overview of lipids, exploring their chemical structure, classification, biological functions, metabolism, and relevance in health and disease. We will also delve into analytical techniques used in lipid research, industrial applications, and future perspectives in this rapidly evolving field.
Table of Contents
Chemical Structure and Classification
Lipids are characterized by their hydrophobic nature, which stems from their molecular structure. The diversity of lipid structures gives rise to a wide range of classifications and properties. Let’s explore the major categories of lipids and their structural features.
1. Fatty Acids
Fatty acids are the simplest lipid molecules, consisting of a hydrocarbon chain with a carboxyl group (-COOH) at one end. They can be classified based on:
a) Chain length:
- Short-chain fatty acids (SCFAs): 2-6 carbon atoms
- Medium-chain fatty acids (MCFAs): 7-12 carbon atoms
- Long-chain fatty acids (LCFAs): 13-21 carbon atoms
- Very long-chain fatty acids (VLCFAs): 22 or more carbon atoms
b) Degree of saturation:
- Saturated fatty acids: No double bonds
- Unsaturated fatty acids: One or more double bonds
- Monounsaturated fatty acids (MUFAs): One double bond
- Polyunsaturated fatty acids (PUFAs): Two or more double bonds
The general formula for a saturated fatty acid is:
CH₃-(CH₂)n-COOH
where n is the number of carbon atoms in the chain minus two.
2. Triglycerides
Triglycerides, also known as triacylglycerols, are the most common form of stored energy in animals and plants. They consist of three fatty acid molecules esterified to a glycerol backbone. The chemical structure of a triglyceride can be represented as:
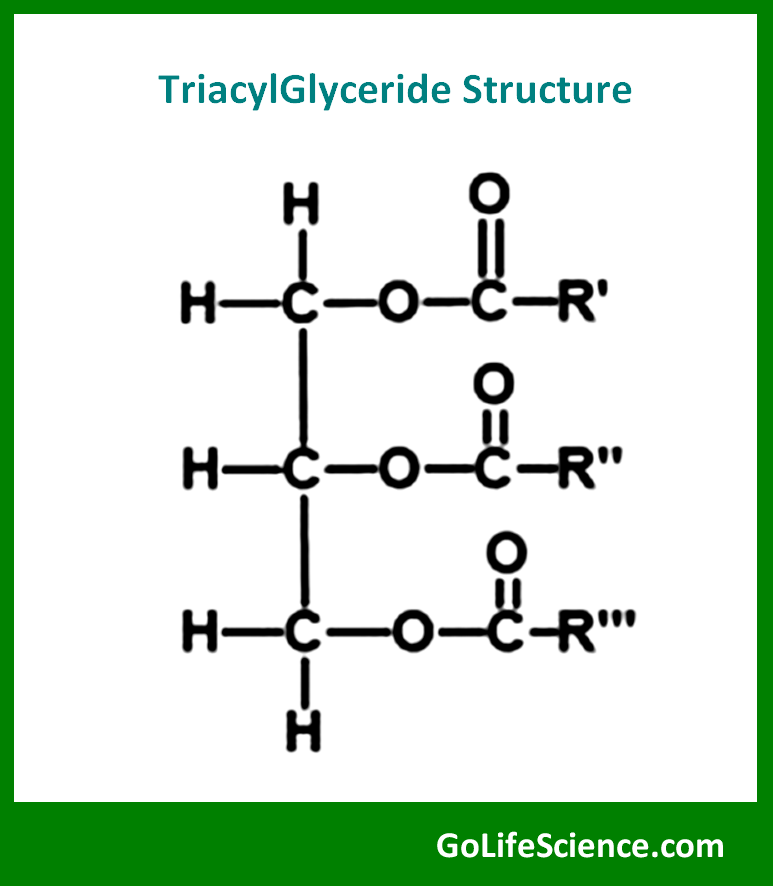
where R₁, R₂, and R₃ represent fatty acid chains.
3. Phospholipids
Phospholipids are major components of cell membranes. They have a hydrophilic head group containing a phosphate and a hydrophobic tail composed of two fatty acid chains. The general structure of a phospholipid is:
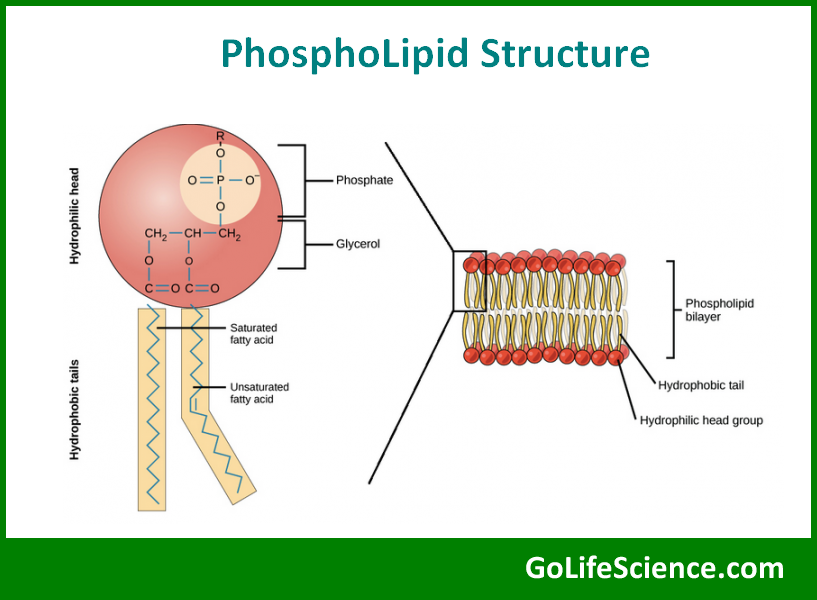
where X represents various molecules that can be attached to the phosphate group, such as choline, ethanolamine, or serine.
4. Steroids
Steroids are a distinct class of lipids characterized by their unique four-ring structure. This tetracyclic ring system, known as the steroid nucleus or gonane, forms the core of all steroid molecules. The steroid nucleus consists of three six-carbon rings (A, B, and C) and one five-carbon ring (D), fused together in a specific configuration.
The basic structure of the steroid nucleus can be represented as:
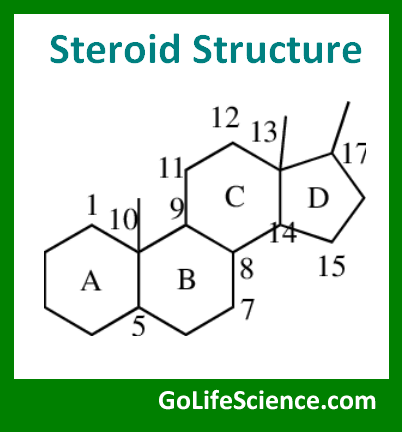
Steroids differ from one another based on the functional groups attached to this core structure and the oxidation state of the rings.
a. Cholesterol
Cholesterol is the most well-known and abundant steroid in animal tissues. It serves as a crucial component of cell membranes and acts as a precursor for various other steroids in the body.
The structure of cholesterol can be represented as:
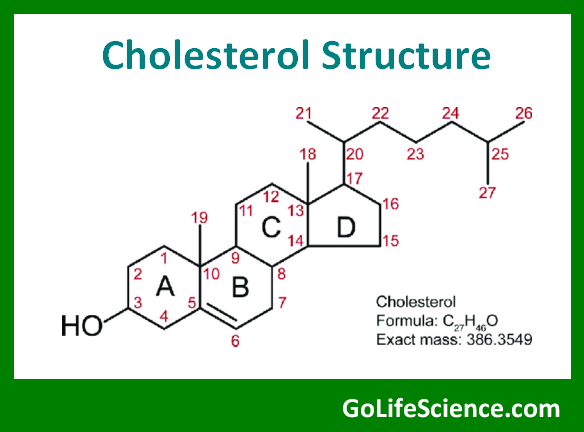
Cholesterol has a molecular formula of C₂₇H₄₆O and a molecular weight of 386.65 g/mol. Key features of its structure include:
- A hydroxyl (-OH) group at carbon-3, which contributes to its amphipathic nature
- A double bond between carbons 5 and 6 in ring B
- An eight-carbon branched hydrocarbon chain attached to carbon-17
b. Other Steroidal Structures
Numerous other biologically important steroids are derived from cholesterol. These include:
- Steroid Hormones
- Glucocorticoids (e.g., cortisol)
- Mineralocorticoids (e.g., aldosterone)
- Sex hormones:
- Androgens (e.g., testosterone)
- Estrogens (e.g., estradiol)
- Progestogens (e.g., progesterone)
- Bile Acids Bile acids, such as cholic acid, are derived from cholesterol and play a crucial role in lipid digestion.
- Vitamin D Vitamin D₃ (cholecalciferol) is synthesized from 7-dehydrocholesterol in the skin upon exposure to UV light.
5. Sphingolipids
Sphingolipids are complex lipids based on the amino alcohol sphingosine. They play important roles in cell signaling and membrane structure. The basic structure of a sphingolipid is:
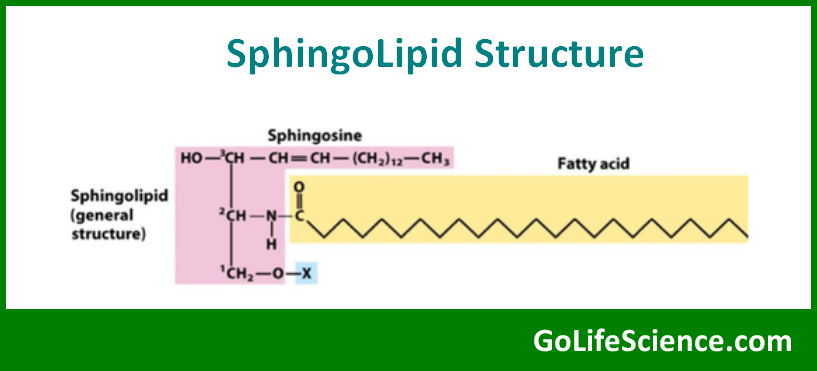
where R and R’ represent various functional groups.
Biological Functions
Lipids perform a wide array of essential functions in living organisms. Let’s explore some of the key roles played by these versatile biomolecules:
1. Energy Storage
Triglycerides are the primary form of energy storage in animals and plants. They provide a concentrated source of energy, yielding about 9 kcal/g compared to 4 kcal/g for carbohydrates and proteins. The energy density of lipids can be represented by the equation:
Energy density (kcal/g) = 9 × mass of lipid (g)
2. Structural Components
Phospholipids and cholesterol are crucial components of cell membranes. The amphipathic nature of phospholipids allows them to form bilayers, which serve as selective barriers between cellular compartments and the external environment. The fluidity of cell membranes is regulated by the ratio of saturated to unsaturated fatty acids and the presence of cholesterol.
3. Signaling Molecules
Many lipids act as signaling molecules, participating in various cellular processes. For example:
- Prostaglandins: Derived from arachidonic acid, they play roles in inflammation and pain perception.
- Steroid hormones: Such as estrogen, testosterone, and cortisol, regulate various physiological processes.
- Phosphatidylinositol derivatives: Act as second messengers in signal transduction pathways.
4. Thermal Insulation
In mammals, subcutaneous fat provides thermal insulation, helping to maintain body temperature. The effectiveness of this insulation can be quantified using the thermal conductivity (k) of fat tissue:
k (fat tissue) ≈ 0.21 W/(m·K)
Compare this to the thermal conductivity of water: k (water) ≈ 0.6 W/(m·K)
The lower thermal conductivity of fat tissue makes it an excellent insulator.
5. Vitamin Absorption
Lipids are essential for the absorption of fat-soluble vitamins (A, D, E, and K) in the intestines. These vitamins are incorporated into mixed micelles formed by bile salts and dietary lipids, facilitating their absorption.
6. Protective Coating
Waxes, a class of lipids, provide protective coatings on leaves, fruits, and animal fur. For example, the waxy cuticle on leaves helps prevent water loss and protects against pathogens.
Lipid Metabolism
Lipid metabolism encompasses the processes by which lipids are synthesized, broken down, and utilized in the body. Understanding these pathways is crucial for comprehending the role of lipids in health and disease.
1. Fatty Acid Synthesis
Fatty acid synthesis occurs primarily in the liver and adipose tissue. The process involves the following key steps:
a) Acetyl-CoA carboxylase catalyzes the formation of malonyl-CoA from acetyl-CoA:
Acetyl-CoA + CO₂ + ATP → Malonyl-CoA + ADP + Pi
b) Fatty acid synthase, a multi-enzyme complex, elongates the fatty acid chain by adding two-carbon units from malonyl-CoA:
Acetyl-CoA + 7 Malonyl-CoA + 14 NADPH + 14 H⁺ → Palmitic acid (16:0) + 7 CO₂ + 8 CoA + 14 NADP⁺ + 6 H₂O
2. Triglyceride Synthesis
Triglycerides are synthesized through the esterification of fatty acids with glycerol. The process involves the following enzymes:
- Glycerol-3-phosphate acyltransferase (GPAT)
- 1-acylglycerol-3-phosphate acyltransferase (AGPAT)
- Phosphatidic acid phosphatase (PAP)
- Diacylglycerol acyltransferase (DGAT)
3. Lipolysis
Lipolysis is the breakdown of triglycerides into fatty acids and glycerol. This process is catalyzed by lipases and is regulated by hormones such as glucagon and epinephrine. The overall reaction can be represented as:
Triglyceride + 3 H₂O → 3 Fatty acids + Glycerol
4. Beta-Oxidation
Beta-oxidation is the primary pathway for fatty acid catabolism, occurring in the mitochondria. The process involves the sequential removal of two-carbon units from the fatty acid chain, generating acetyl-CoA molecules. The general equation for the oxidation of a saturated fatty acid with n carbon atoms is:
CnH₂nO₂ + (n/2 – 1) O₂ + (n/2 – 1) CoA-SH + (n/2 – 1) H₂O → n/2 CH₃CO-SCoA + (n/2 – 1) FADH₂ + (n/2 – 1) NADH + (n/2 – 1) H⁺
5. Ketone Body Synthesis
During periods of prolonged fasting or carbohydrate restriction, the liver produces ketone bodies from acetyl-CoA derived from fatty acid oxidation. The three main ketone bodies are:
- Acetoacetate
- Beta-hydroxybutyrate
- Acetone
The synthesis of ketone bodies involves the following key enzymes:
- 3-hydroxy-3-methylglutaryl-CoA synthase (HMG-CoA synthase)
- HMG-CoA lyase
- Beta-hydroxybutyrate dehydrogenase
6. Cholesterol Synthesis
Cholesterol synthesis is a complex process involving multiple enzymes. The rate-limiting step is catalyzed by HMG-CoA reductase:
HMG-CoA + 2 NADPH + 2 H⁺ → Mevalonate + 2 NADP⁺ + CoA-SH
This reaction is a target for statin drugs, which are used to lower cholesterol levels.
Lipids in Health and Disease
Lipids play crucial roles in maintaining health, but imbalances in lipid metabolism can contribute to various diseases. Here, we explore some key aspects of lipids in health and disease:
1. Cardiovascular Disease
Lipid profiles are important indicators of cardiovascular health. The National Cholesterol Education Program (NCEP) provides guidelines for optimal lipid levels:
| Lipid Parameter | Optimal Level (mg/dL) |
|---|---|
| Total Cholesterol | < 200 |
| LDL Cholesterol | < 100 |
| HDL Cholesterol | < 60 |
| Triglycerides | < 150 |
Elevated levels of LDL cholesterol and triglycerides, along with low levels of HDL cholesterol, are associated with an increased risk of atherosclerosis and cardiovascular disease.
2. Obesity
Excessive accumulation of triglycerides in adipose tissue leads to obesity. The body mass index (BMI) is often used to classify weight status:
BMI = Weight (kg) / (Height (m))²
| BMI Range | Weight Status |
|---|---|
| < 18.5 | Underweight |
| 18.5 – 24.9 | Normal |
| 25.0 – 29.9 | Overweight |
| ≥ 30.0 | Obese |
3. Diabetes
Insulin resistance, a key feature of type 2 diabetes, is closely linked to lipid metabolism. Elevated levels of free fatty acids can impair insulin signaling and glucose uptake in tissues.
4. Neurodegenerative Diseases
Lipids are crucial components of the nervous system. Alterations in lipid metabolism have been implicated in neurodegenerative diseases such as Alzheimer’s and Parkinson’s disease.
5. Cancer
Certain lipids and their metabolites have been associated with cancer progression. For example, elevated levels of prostaglandin E2 (PGE2) have been observed in various types of cancer.
6. Genetic Disorders of Lipid Metabolism
Several genetic disorders affect lipid metabolism, including:
- Familial hypercholesterolemia
- Gaucher disease
- Tay-Sachs disease
- Niemann-Pick disease
These disorders highlight the importance of proper lipid metabolism for overall health.
Analytical Techniques for Lipid Research
Advances in analytical techniques have revolutionized lipid research. Some key methods include:
1. Chromatography
- Thin-layer chromatography (TLC)
- Gas chromatography (GC)
- High-performance liquid chromatography (HPLC)
2. Mass Spectrometry
- Electrospray ionization mass spectrometry (ESI-MS)
- Matrix-assisted laser desorption/ionization (MALDI-MS)
3. Nuclear Magnetic Resonance (NMR) Spectroscopy
NMR provides detailed structural information about lipid molecules.
4. Lipidomics
Lipidomics is the comprehensive analysis of lipid species in biological systems, often combining chromatography and mass spectrometry techniques.
Lipids in Industry and Technology
Lipids have numerous applications beyond their biological roles:
1. Food Industry
- Emulsifiers
- Flavor enhancers
- Texture modifiers
2. Cosmetics and Personal Care
- Moisturizers
- Emollients
- Surfactants
3. Biofuels
Lipids, particularly triglycerides from plant oils, can be converted into biodiesel through transesterification:
Triglyceride + 3 Methanol ⇌ 3 Fatty Acid Methyl Esters (Biodiesel) + Glycerol
4. Pharmaceuticals
Lipids are used in drug delivery systems, such as liposomes and solid lipid nanoparticles.
Future Perspectives in Lipid Research
The field of lipid research continues to evolve, with several exciting areas of investigation:
- Personalized lipid profiling for disease risk assessment and treatment
- Development of lipid-based nanomaterials for targeted drug delivery
- Exploration of the gut microbiome’s role in lipid metabolism
- Investigation of lipid signaling networks in cellular processes
- Advancement of lipidomics technologies for high-throughput analysis
Frequently Asked Questions (FAQS) on Lipids
What are lipids?
Lipids are a diverse group of organic compounds that are insoluble in water but soluble in nonpolar solvents. They include fats, oils, waxes, phospholipids, and steroids.
What is the basic structure of a lipid?
The basic structure of a lipid typically consists of a glycerol molecule bonded to fatty acids. The fatty acids can be saturated or unsaturated, affecting the lipid’s properties and functions.
What are the main functions of lipids in the body?
Lipids serve several crucial functions, including energy storage, forming cell membranes, and acting as signaling molecules. They also provide insulation and protection for organs.
How are lipids classified?
Lipids are classified into several categories, including triglycerides, phospholipids, sterols, and waxes. Each type has distinct structures and functions within biological systems.
What role do phospholipids play in cells?
Phospholipids are essential components of cell membranes. They form a bilayer that acts as a barrier, regulating the entry and exit of substances in and out of the cell.