
The normal cell consists of 5 to 10 times as much RNA (mRNAs, rRNAs and also tRNAs) as DNA. For that reason, most of the nucleotide biosynthesis has as its objective the manufacturing of rNTPs.
Nonetheless, since multiplying cells should reproduce their genomes, the manufacturing of dNTPs is likewise needed.

How are the Deoxyribonucleotides synthesizing in the cell?
This procedure starts with the decrease of rNDPs, complied with by phosphorylation to produce the dNTPs.
The phosphorylation of dNDPs to dNTPs is catalyzed by the exact same nucleoside diphosphate kinases that phosphorylate ribonucleoside diphosphate to riboNucleotide TriPhosphate by using one ATP molecule.
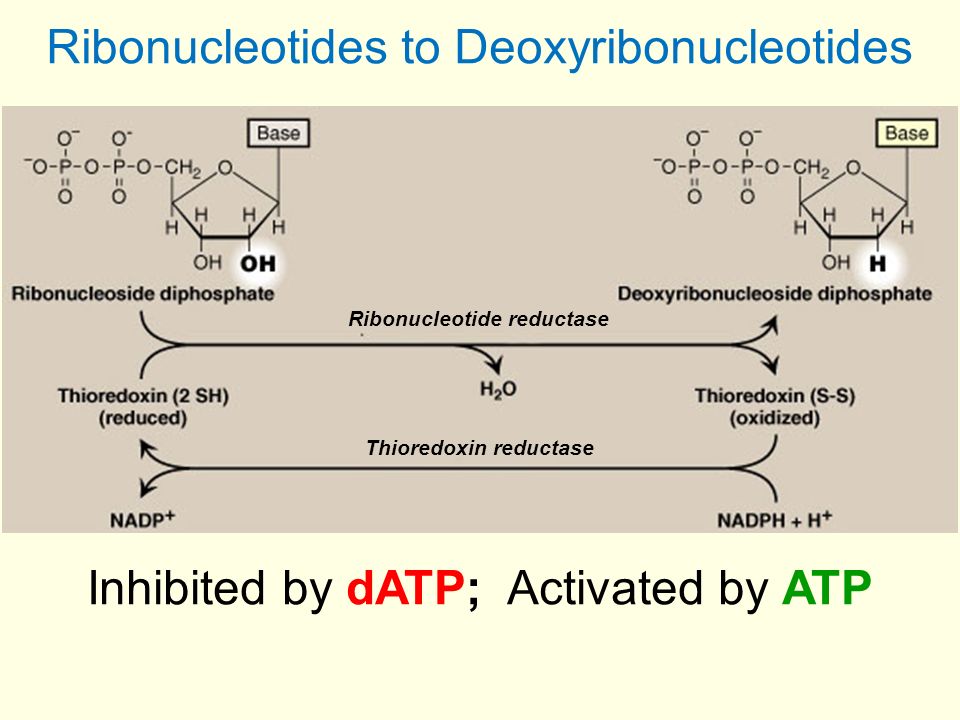
Ribonucleotide reductase (RR) is a multifunctional enzyme that contains redox-active thiol groups for the transfer of electrons throughout the decrease responses.
When decreasing the Ribonucleotide Phosphate to a deoxyriboNucleotide DiPhosphate, in the presence of RR enzyme.
RR is decreased subsequently, by either thioredoxin or glutaredoxin. The final source of the electrons is NADPH.
The electrons are shuttled with a complicated collection of actions including enzymes that restore the lower types of thioredoxin or glutaredoxin.
These enzymes are thioredoxin reductase and also glutathione reductase specifically.
The Regulation of dNTP Development
Ribonucleotide reductase is the only enzyme made use of in the formation of all the deoxyribonucleotides.
For that reason, its task and also substratum uniqueness need to be securely controlled to make certain well-balanced manufacturing of all 4 of the dNTPs needed for DNA duplication.
Such law happens by binding of nucleoside triphosphate effects to either the task websites or the uniqueness websites of the enzyme complicated.
The task websites bind either ATP or dATP with reduced fondness, whereas the uniqueness websites bind ATP, dATP, dGTP, or dTTP with high fondness.
The binding of ATP at task websites causes enhanced enzyme tasks, while the binding of dATP hinders the enzyme.
The binding of nucleotides at uniqueness websites efficiently permits the enzyme to discover the family member’s wealth of the 4 dNTPs and also to change its fondness for the much less plentiful dNTPs.
In order to attain an equilibrium of manufacturing. thioredoxin reductase and also glutathione reductase specifically.
