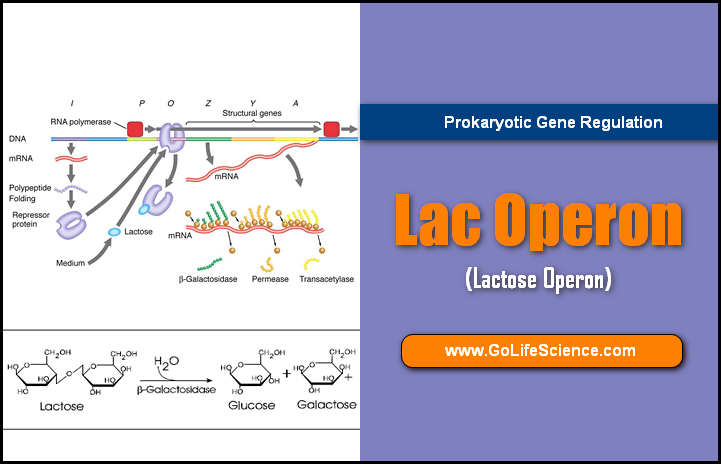
Euchromatin is a lightly packed form of chromatin (DNA, RNA, and protein) that is rich in gene concentration and is often (but not always) under active transcription.
Unlike Heterochromatin, it is found in both cells with nuclei (eukaryotes) and cells without nuclei (prokaryotes).
Euchromatin refers to the loosely packed form of chromatin that contains frequently expressed and transcribed genes. In contrast to the condensed, tightly packed heterochromatin, Euchromatin is more accessible to regulatory transcription factors and the transcriptional machinery. It allows for active gene transcription and expression.
Euchromatin is essential for regulating gene expression programs that control cellular phenotype and function. Dynamic changes between Euchromatin and heterochromatin are critical for cell differentiation, response to environmental stimuli, and embryonic development. Dysfunctions in euchromatin regulation are linked to diseases like cancer.

This article will explore the molecular composition, dynamic nature, and techniques to study Euchromatin. The vital roles of Euchromatin in gene regulation, human health, and disease will also be discussed.
Table of Contents
The Molecular Composition of Euchromatin and Proteins
The organization of chromatin into euchromatin and heterochromatin is a fundamental aspect of genome structure in eukaryotic cells. While euchromatin is associated with active gene expression, constitutive heterochromatin and facultative heterochromatin represent the repressed and compacted regions of the genome.
Visualizing these chromatin domains often requires special staining techniques when observing chromosome structures under a microscope.
Euchromatin and heterochromatin represent two distinct forms of chromatin, each with specific roles inside the cell nucleus. The differences between euchromatin and heterochromatin lie in their structure and transcriptional activity.
Euchromatin is often associated with active gene expression, whereas heterochromatin tends to be more condensed and transcriptionally inactive. This spatial organization of chromatin within interphase nuclei plays a crucial role in regulating gene expression throughout the cell cycle.
In the intricate world of cellular biology, the molecular composition of Euchromatin is a tapestry woven from a variety of essential components, each with a unique role to play in the orchestration of gene expression. Let’s explore these elements in detail.
Histones and DNA
The basic unit of chromatin is the nucleosome, which consists of DNA wrapped around a histone protein core. Histone proteins regulate euchromatin formation through post-translational modifications that control DNA accessibility and packing.
DNA and Histone Proteins: One of the key features of euchromatin is its unfolded structure, reminiscent of ‘beads on a string,’ where DNA is wrapped around histone proteins. This structure, maintained by histone modifications such as acetylation of histone H3K27, allows for easy access of transcription factors, thereby facilitating the activation of transcription.
The acetylation of histones is a crucial step in the regulation of chromatin, ensuring that the transcription factories within euchromatin remain productive.
Role of Histone Proteins
Histones, often called the “spools” of DNA, are fundamental players in Euchromatin’s molecular architecture. These proteins are like molecular caretakers, meticulously wrapping and protecting the delicate threads of genetic information. But their role goes beyond mere packaging; histones actively participate in the regulation of gene expression.
Histones serve as dynamic platforms for epigenetic marks, influencing whether genes are silenced or activated. These marks, in the form of chemical modifications, can include acetylation, methylation, phosphorylation, and more. By “decorating” histones with these marks, cells can fine-tune the accessibility of DNA, essentially signaling whether a gene should remain silent or be awakened for transcription. This orchestration of epigenetic modifications on histones is a key determinant of Euchromatin’s dynamic nature.
DNA Accessibility and Packaging
Euchromatin’s hallmark feature is its accessibility. Unlike heterochromatin, which tightly packages DNA into a condensed, less accessible form, Euchromatin offers genes a more open, permissive environment. This accessibility is crucial for various cellular processes, such as DNA replication, repair, and, most notably, gene transcription.
Within Euchromatin, DNA is not confined behind locked doors but is readily accessible to various cellular machinery. This accessibility allows the transcriptional machinery—RNA polymerases and associated factors—to access genes easily, facilitating the process of gene expression. The dynamic interplay between histones and DNA packaging ensures that genes housed within Euchromatin are poised for action, ready to respond to the cellular cues that trigger their expression.
Epigenetic Modifications
Epigenetic modifications of DNA and histones are key determinants of chromatin structure that influence euchromatin formation.
The realm of epigenetic modifications further underscores Euchromatin’s dynamic nature. Epigenetics, a field at the intersection of genetics and molecular biology, explores the heritable changes in gene expression that occur without alterations to the underlying DNA sequence. Two primary actors in this context are DNA methylation and histone modifications.
DNA Methylation
DNA methylation is a biochemical process by which methyl groups are added to the DNA molecule. This modification typically occurs at specific regions known as CpG islands, where a cytosine (C) nucleotide is followed by a guanine (G) nucleotide in the DNA sequence. DNA methylation can serve as a molecular switch, silencing gene expression. When CpG islands in gene promoters are methylated, the associated genes are often turned off, contributing to the fine-tuned regulation of gene activity within Euchromatin.
Histone Modifications
Histone modifications are a diverse array of chemical changes that occur on histone proteins, decorating them with various “tags.” These tags can include acetyl, methyl, or phosphate groups. Depending on the type and location of these modifications, they can either enhance or inhibit gene expression.
For example, histone acetylation promotes gene expression by loosening the histone-DNA interaction and making the DNA more accessible to transcription factors. On the other hand, histone methylation can have both activating and repressive effects, depending on which specific amino acids on the histone proteins are modified and the degree of methylation.
The intricate interplay between DNA methylation and histone modifications within Euchromatin is a dynamic process that allows cells to fine-tune gene expression in response to developmental cues, environmental signals, and cellular needs.
Facultative heterochromatin is assembled on developmentally regulated loci, which can switch between transcriptionally active and inactive chromatin states during development and differentiation. As a result, not all cell types will have an identical pattern of facultative heterochromatin distribution in the genome. Facultative heterochromatin is regulated by polycomb group (PcG) proteins that impart histone 3 lysine 27 trimethylation (H3K27me3) marks.
Chromatin Remodeling Complexes
1. ATP-Dependent Remodeling
Chromatin remodeling complexes are the architects of Euchromatin’s structural changes. These molecular machines use the energy currency of cells, ATP (adenosine triphosphate), to reconfigure the positioning of nucleosomes—the fundamental units of chromatin. By sliding, ejecting, or restructuring nucleosomes, these complexes can make specific euchromatin regions more or less accessible to the transcriptional machinery.
ATP-dependent remodeling complexes are particularly fascinating because they can directly impact the accessibility of genes within Euchromatin. By altering the positioning of nucleosomes, they can expose previously hidden regions of DNA, allowing for gene activation. Conversely, they can compact chromatin, rendering genes less accessible and effectively silencing them.
2. Role in Euchromatin Formation
The actions of chromatin remodeling complexes are instrumental in shaping Euchromatin. They are responsible for maintaining Euchromatin’s open and dynamic state, ensuring that genes housed within it can be activated or silenced in response to cellular cues. These complexes sculpt Euchromatin’s landscape, chiseling out the intricate patterns that dictate gene expression.
In summary, the molecular composition of Euchromatin is a dynamic interplay of histone proteins, DNA packaging, epigenetic modifications, and chromatin remodeling complexes. Together, these components choreograph the symphony of gene expression, allowing cells to fine-tune which genes are active and which remain silent—a pivotal aspect of cellular function and differentiation.
Traditionally, interphase chromatin is classified as either euchromatin or heterochromatin, depending on its level of compaction.
Heterochromatin Formation: Unraveling the DNA Binding Mystery
Heterochromatin formation represents a critical aspect of chromatin organization within the cell nucleus. Understanding how these condensed regions bind to the DNA is essential for unraveling the intricate mechanisms behind gene regulation and genome stability.
At the heart of heterochromatin formation lies the ability of specific proteins and protein complexes to bind to the DNA with precision. This binding process leads to the compaction of chromatin into a transcriptionally repressive state, silencing genes and restricting access to the underlying DNA sequences.
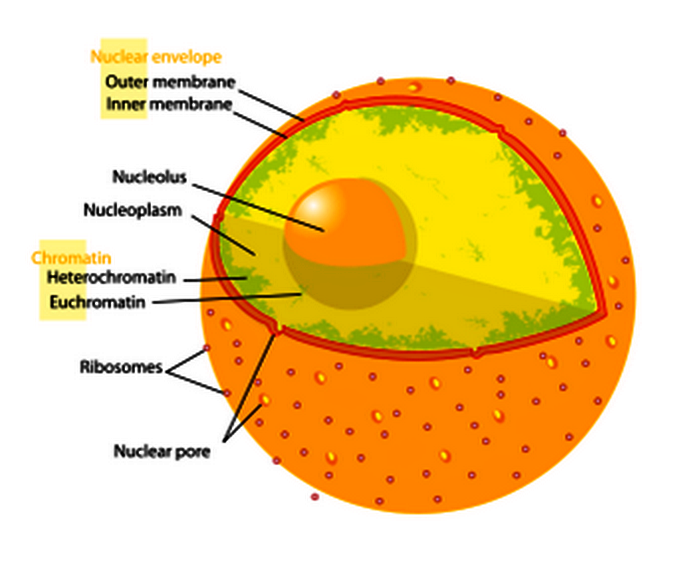
These proteins play a crucial role in creating an environment where gene expression is suppressed, contributing to maintaining genomic integrity.
The process of heterochromatin formation is not uniform throughout the genome. Specific DNA sequences and regions are targeted for binding by these regulatory proteins, ensuring that only select areas are tightly packed into heterochromatin.
This selective binding is a testament to the complexity of gene regulation, allowing for precise control over which genes are turned on and off in response to cellular cues.
In studying heterochromatin formation, researchers aim to decipher the molecular interactions underlying proteins’ binding to DNA. By uncovering the intricacies of this process, we gain insights into the regulation of gene expression, the silencing of repetitive elements, and the maintenance of genome stability.
This knowledge contributes to our understanding of fundamental cell biology and holds promise for potential therapeutic interventions in diseases where heterochromatin regulation goes awry.
Appearance :
In general, It appears as light-colored bands when stained in G-banding and observed under an optical microscope, in contrast to Heterochromatin, which stains darkly. This lighter staining is due to the less compact structure of euchromatin.
The basic structure of euchromatin is an elongated, open, 10nm microfibril, as noted by electron microscopy.
In prokaryotes, this is the only form of chromatin present; this indicates that the Heterochromatin structure evolved later along with the nucleus, possibly as a mechanism to handle increasing genome size.
Dynamic Nature of Euchromatin
Chromatin, often described as the “active Euchromatin”, is far from static; it’s a dynamic entity that adapts and responds to various cues within the cellular environment. This dynamic nature of Euchromatin influences diverse aspects of cellular biology and genetics.
Euchromatin vs. Heterochromatin
- Euchromatin vs. heterochromatin: Euchromatin’s dynamism is highlighted when compared to its counterpart, heterochromatin, known for its densely packed, condensed chromatin structure, resulting in transcriptional inactivity.
- Structural differences: Euchromatin is more open and accessible, while heterochromatin is tightly compacted. But what sets them apart beyond their structural distinctions?
- Euchromatin as a hub for gene expression: Euchromatin is not merely an “open for business” version of chromatin; it also serves as a hub for active gene expression. Genes within euchromatin are poised to respond to cellular cues that trigger their transcription.
- Contrasting gene activity: This starkly contrasts with heterochromatin, where genes are typically silenced and inaccessible.
- Euchromatin’s dynamic nature: Euchromatin’s dynamic properties make it a hotspot for various cellular activities, including DNA replication, repair, and, most importantly, gene transcription.
Euchromatin and Cell Differentiation
1. Embryonic Development
- Euchromatin’s role in embryonic development: Euchromatin’s dynamic properties are pivotal during embryonic development, where it guides the differentiation of a single fertilized egg cell into various cell types to form a complex organism.
- Precision in cell fate determination: During early embryogenesis, euchromatin plays an active role in defining cell fate. Specific genes within euchromatin are activated or silenced to ensure each cell follows its designated differentiation path, contributing to the formation of various tissues and organs and the development of the body’s intricate structure.
- Importance in early development: Euchromatin is particularly crucial in the early stages of development, as seen in mouse embryonic stem cells. These cells need to swiftly switch between transcriptionally active and inactive states to give rise to various cell types, and euchromatin’s neutral jurisdictional claims regarding transcription facilitate this dynamic gene expression required for differentiation.
2. Tissue-Specific Gene Expression
- Euchromatin’s role in tissue-specific gene expression: Euchromatin ensures tissue-specific gene expression, allowing genes relevant to a particular cell type to be activated while keeping others silent.
- Specialized gene expression in different cell types: Muscle cells activate genes responsible for muscle contraction, while nerve cells activate genes related to neuron function, demonstrating how euchromatin regulates cell-specific gene expression.
- Euchromatin’s dynamic regulation: Euchromatin dynamically regulates gene expression to ensure the right genes are activated in the appropriate cells, contributing to the proper functioning of multicellular organisms.
- Spatial organization during cell division: Chromatin is organized into distinct chromosome territories during cell division, segregating euchromatic and heterochromatic regions to maintain the integrity of the human genome.
- Protein complexes in genome stability: Certain protein complexes play a role in binding and maintaining heterochromatin, emphasizing its importance in genome stability.
- Principles of chromatin folding: Understanding principles like phase separation is essential for comprehending how different genomic regions contribute to gene regulation and how euchromatin and heterochromatin interact with the transcription machinery.
- Advanced techniques for visualization: Researchers use advanced techniques to visualize and dissect chromosome structure, shedding light on the transcription process within euchromatic regions.
- Central role of euchromatin and heterochromatin: Euchromatin and heterochromatin play a central role in gene expression regulation and the structure of the genome.
- Interplay of core histones, protein complexes, and RNA polymerase II: The interplay of these components within genomic regions highlights their pivotal roles in the transcriptional landscape.
- Deeper insights into gene expression dynamics: As our understanding of chromosome territories and chromatin compaction principles advances, we gain deeper insights into the dynamic realm of gene expression in the human genome (Creative Commons Attribution).
Euchromatin and Environmental Influences
1. Response to External Stimuli
Euchromatin’s adaptability extends beyond developmental processes and affects how cells respond to external stimuli. Cells constantly encounter environmental changes, and their ability to adjust gene expression accordingly is crucial for survival.
Euchromatin’s openness allows for rapid changes in gene expression in response to external cues. When faced with environmental stressors, such as temperature fluctuations or exposure to toxins, cells can activate specific genes within Euchromatin to mount a protective response. This ability to fine-tune gene expression is a testament to the versatility of Euchromatin in helping cells adapt to their surroundings.
2. Role in Disease
Aberrations in euchromatin structure and function have been linked to various diseases, including cancer. Euchromatin’s dynamic nature can be both a blessing and a curse in the context of disease.
In cancer, for example, the dysregulation of Euchromatin can lead to the abnormal activation of genes that promote uncontrolled cell growth. Understanding these connections between Euchromatin and disease is a focus of intense research, as it promises to develop novel therapeutic interventions that target the specific genes within Euchromatin responsible for disease progression.
Euchromatin’s dynamic nature is a central theme in the intricate world of cellular biology. It guides embryonic development, fine-tunes tissue-specific gene expression, enables cells to respond to external stimuli, and plays a pivotal role in the development of diseases. This dynamic aspect of Euchromatin underscores its importance as a focal point of genetics research and provides a rich source of insight into the complexities of cellular life.
Techniques to Study Euchromatin
Understanding Euchromatin at a molecular level requires specialized techniques that allow researchers to peer into its intricate structure and unravel its secrets. These techniques shed light on the dynamic world of Euchromatin, revealing its role in gene expression, epigenetics, and chromatin organization.
A. Chromatin Immunoprecipitation (ChIP)
Chromatin Immunoprecipitation, often abbreviated as ChIP, is a powerful tool in the arsenal of molecular biologists. It enables researchers to investigate the interactions between DNA and specific proteins within Euchromatin. Here’s how it works:
- Crosslinking: The process begins by chemically crosslinking the DNA and associated proteins within cells, essentially freezing them in their current state. This step preserves the interactions that are occurring in the Euchromatin.
- Cell Lysis: The cell membranes are then disrupted, releasing the cellular contents, including the chromatin.
- Immunoprecipitation: Antibodies specific to the protein of interest are added to the chromatin mixture. These antibodies bind to their target proteins, fishing them from the chromatin soup.
- DNA Extraction: After the immunoprecipitation step, the DNA associated with the protein of interest is isolated. This DNA is the portion of Euchromatin that interacts with the target protein.
- Analysis: The isolated DNA can be analyzed using various methods, including PCR or high-throughput sequencing. It allows researchers to identify specific regions of Euchromatin that interact with the protein of interest.
ChIP is a versatile technique that has numerous applications. It can be used to study the binding of transcription factors to Euchromatin, investigate histone modifications, and explore how epigenetic marks influence gene expression within Euchromatin.
Understanding the spatial organization of chromatin, whether it is inside the cell nucleus, or how heterochromatin segregates from euchromatin, has been a subject of extensive research. Techniques such as fluorescence in situ hybridization have shed light on the distribution of heterochromatin and euchromatin on interphase chromosomes, revealing their distinct roles in gene regulation.
B. DNase I Hypersensitivity Assay
The DNase I Hypersensitivity Assay is a technique that provides insights into the accessibility of euchromatin regions. It helps identify areas within Euchromatin that are more “open” and thus more likely to be actively transcribed. Here’s how it works:
- Treatment with DNase I: DNase I is an enzyme that cleaves DNA at locations where it is not tightly packaged. DNase I preferentially digests the less condensed regions when applied to cells or isolated chromatin, leaving behind the more accessible Euchromatin.
- DNA Isolation: After DNase I treatment, the DNA is isolated from the cells or chromatin.
- Analysis: The isolated DNA is then analyzed to identify regions more susceptible to DNase I digestion. These regions are considered “hypersensitive” and indicate euchromatin regions actively participating in gene expression.
The DNase I Hypersensitivity Assay provides a snapshot of euchromatin accessibility and is valuable for identifying regulatory elements, and active transcription start sites within Euchromatin. It is a crucial technique for understanding the functional regions within Euchromatin.
C. Hi-C and 3C Technologies
Hi-C (High-throughput Chromosome Conformation Capture) and 3C (Chromosome Conformation Capture) Technologies are revolutionary techniques that provide insights into the three-dimensional organization of Euchromatin within the nucleus. They help us understand how genes, regulatory elements, and euchromatin regions are spatially arranged and interact.
Here’s how these techniques work:
- Crosslinking: Similar to ChIP, the first step involves crosslinking chromatin within cells to “freeze” their spatial organization.
- Restriction Enzyme Digestion: The chromatin is then digested with a restriction enzyme, which cuts the DNA at specific recognition sites. This step creates small DNA fragments, each representing a piece of Euchromatin.
- Ligation: The DNA fragments are then ligated together, effectively joining distant pieces of Euchromatin that were in close physical proximity within the nucleus.
- Analysis: The ligated DNA fragments are sequenced, and the data is analyzed to construct a three-dimensional map of how euchromatin regions are spatially organized within the nucleus. This information helps researchers understand how genes interact with each other and with regulatory elements within Euchromatin.
Hi-C and 3C Technologies have revolutionized our understanding of Euchromatin by revealing its intricate three-dimensional structure of euchromatin. Researchers can now explore how genes are regulated based on their spatial proximity within Euchromatin, providing valuable insights into gene expression control.
In conclusion, these techniques to study Euchromatin offer a multifaceted approach to unraveling its complexity. They enable researchers to investigate the interactions, accessibility, and spatial organization of euchromatin regions, shedding light on the fundamental processes that govern gene expression and regulation within cells.
Euchromatin and Human Health
The influence of Euchromatin on human health extends far beyond the confines of cellular biology. It plays a pivotal role in developing and progressing various health-related conditions and holds promise as a target for therapeutic interventions. Let’s delve into the multifaceted relationship between Euchromatin and human health.
Implications in Cancer
a. Cancer and Euchromatin
Euchromatin’s role in the development and progression of cancer is an area of intense research. The dynamic nature of Euchromatin can be both a boon and a bane when it comes to cancer.
On one hand, Euchromatin’s permissiveness to gene expression can lead to the activation of genes that promote uncontrolled cell growth—the hallmark of cancer. Aberrant regulation of genes within Euchromatin can result in the unchecked proliferation of cancer cells.
Understanding these connections between Euchromatin and cancer is crucial for developing targeted therapies to modulate gene expression within cancer cells, potentially slowing or halting their growth.
b. Epigenetic Changes in Cancer
Euchromatin is particularly vulnerable to epigenetic alterations in cancer. DNA methylation patterns can become dysregulated, leading to the silencing of tumor-suppressor genes within Euchromatin. Additionally, histone modifications within Euchromatin can create a permissive environment for oncogenes, genes that promote cancer development, to become hyperactivated.
Genetic Disorders and Euchromatin Aberrations
Euchromatin aberrations can also contribute to the development of genetic disorders. In some cases, mutations or structural changes within Euchromatin can disrupt the normal regulation of gene expression.
For example, certain genetic disorders are associated with mutations in the epigenetic machinery that governs Euchromatin. These mutations can lead to widespread changes in gene expression, causing a cascade of physiological disruptions. Understanding how euchromatin aberrations contribute to genetic disorders is critical for diagnosis and potential therapeutic interventions.
Therapeutic Potential: Targeting Euchromatin for Therapy
Euchromatin’s dynamic nature makes it an attractive target for therapeutic interventions. Researchers are exploring ways to manipulate the accessibility and activity of Euchromatin genes to treat various health conditions.
- Cancer Therapies: In the realm of cancer treatment, therapies that target Euchromatin are being developed. These therapies aim to modulate the activity of specific genes within Euchromatin to slow or halt cancer cell growth. By exploiting the dynamic nature of Euchromatin, researchers are exploring novel avenues for cancer treatment.
- Epigenetic Therapies: Euchromatin is intricately linked to epigenetic regulation. Epigenetic therapies, such as drugs that modify DNA methylation patterns or histone modifications, hold promise for treating diseases associated with euchromatin aberrations, including cancer and genetic disorders.
- Gene Regulation: Euchromatin’s role in gene regulation can be harnessed for therapeutic purposes. Researchers are exploring ways to activate or suppress specific genes within Euchromatin to treat a wide range of conditions, from metabolic disorders to neurodegenerative diseases.
- Profound impact on human health: Euchromatin’s influence on human health is significant and multifaceted, with connections to the development and progression of diseases, such as cancer and genetic disorders.
- Potential for therapeutic interventions: Euchromatin’s dynamic nature provides opportunities for therapeutic interventions that could potentially revolutionize the field of medicine.
- Research advancements and healthcare improvement: As research in this area progresses, there is hope that targeting euchromatin for therapeutic purposes could lead to improved healthcare and more effective disease management.
- Euchromatin’s characteristics: Euchromatin, known for its relaxed and unfolded nucleosome structure, serves as a platform for the transcription machinery, including RNA polymerase II.
- Role of RNA polymerase: RNA polymerase’s pivotal role in euchromatin is essential for facilitating the transcription of genes crucial for cell biology and various cellular processes.
Functions of Chromatin:
- It participates in the active transcription of DNA to mRNA products.
- The unfolded structure allows gene regulatory proteins and RNA polymerase complexes to bind to the DNA sequence, which can then initiate the transcription process.
- Not all euchromatin is necessarily transcribed, but in general, that which is not is transformed into Heterochromatin to protect the genes while they are not in use.
- There is, therefore, a direct link between how actively productive a cell is and the amount of this type of chromatin that can be found in its nucleus.
- The cell is thought to use transformation from euchromatin into Heterochromatin to control gene expression and replication since such processes behave differently on densely compacted chromatin, known as the “accessibility hypothesis.”
- One example of constitutive euchromatin is always turned into housekeeping genes, which code for the proteins needed for essential functions of cell survival.
Final words of Euchromatin from Chromatin
- Euchromatin allows gene transcription through an open chromatin state and is pivotal for controlling cell identity and function.
- The euchromatin landscape is highly dynamic and responsive, adapting to facilitate developmental and physiological needs.
- Understanding euchromatin biology will provide insights into human health and open new therapeutic avenues.
- Ongoing research continues to reveal the complexities of euchromatin regulation and its impacts on the genome’s functioning.
In summary, euchromatin is the dynamic realm of gene expression, offering a welcoming environment for transcription factors, and serving as a critical player in the regulation of gene activity. Its crucial role during embryonic development, especially in embryonic stem cells, underscores the significance of understanding the differences between euchromatin and heterochromatin and the transcriptional processes that take place within these chromatin domains.