The salvage pathway of pyrimidine nucleotides is a crucial metabolic process in cellular biology. This pathway allows cells to recycle preformed nucleosides and bases, conserving energy and resources. In this article, we’ll explore the intricacies of this pathway, its significance, and its role in cellular metabolism. let us dive into details on the Pyrimidine Salvage Pathway right now.
What are Pyrimidine Nucleotides?
Before delving into the salvage pathway, let’s first understand pyrimidine nucleotides:
Pyrimidine nucleotides are essential components of nucleic acids (DNA and RNA). They consist of:
- A nitrogenous base (cytosine, thymine, or uracil)
- A pentose sugar (ribose or deoxyribose)
- One or more phosphate groups
The three primary pyrimidine bases are:

- Cytosine (C): Found in both DNA and RNA
- Thymine (T): Found only in DNA
- Uracil (U): Found only in RNA (replaces thymine)
Importance of Nucleotide Metabolism
Nucleotide metabolism is vital for cellular function and survival. It involves two main pathways:
- De novo synthesis: The creation of nucleotides from simple precursor molecules
- Salvage pathway: The recycling of preformed nucleosides and bases
The salvage pathway is particularly important because it:
- Conserves energy
- Reduces the need for de novo synthesis
- Allows the cell to utilize exogenous nucleosides and bases
- Plays a crucial role in maintaining the nucleotide pool
What is the salvage pathway of pyrimidine nucleotides synthesis?
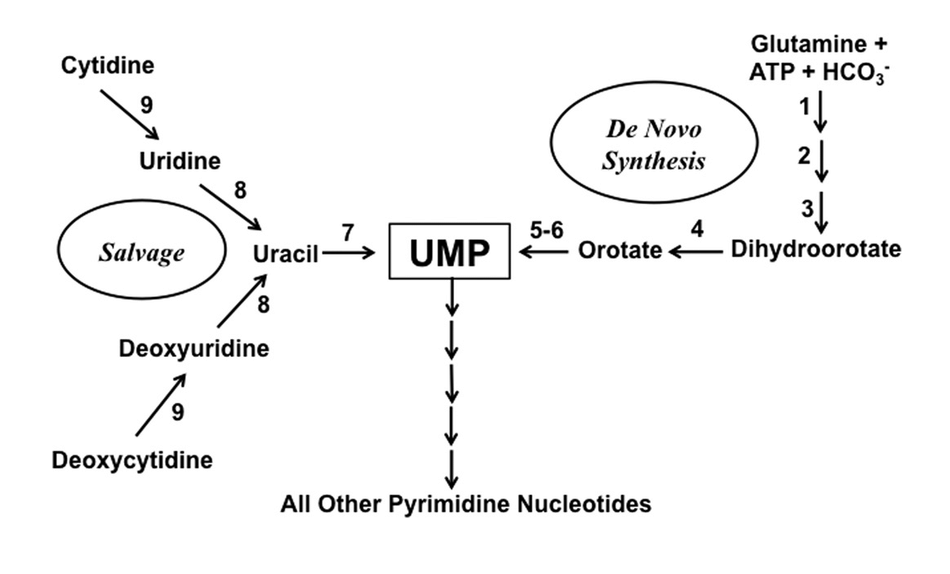
1. Nucleoside Transport
The first step in the salvage pathway involves the transport of nucleosides across the cell membrane. This process is facilitated by two types of transporters:
- Concentrative Nucleoside Transporters (CNTs): These are sodium-dependent and can transport nucleosides against their concentration gradient.
- Equilibrative Nucleoside Transporters (ENTs): These facilitate bidirectional transport of nucleosides down their concentration gradient.
The general equation for nucleoside transport can be represented as:
Nucleoside (extracellular) ⇌ Nucleoside (intracellular)
2. Nucleoside Phosphorylation
Once inside the cell, nucleosides undergo phosphorylation to form nucleoside monophosphates. This process is catalyzed by specific kinases:
- Uridine-Cytidine Kinase (UCK): Uridine + ATP ⇌ UMP + ADP Cytidine + ATP ⇌ CMP + ADP
- Thymidine Kinase (TK): Thymidine + ATP ⇌ TMP + ADP
- Deoxycytidine Kinase (dCK): Deoxycytidine + ATP ⇌ dCMP + ADP
3. Nucleobase Salvage
Free pyrimidine bases can also be salvaged through the action of phosphoribosyltransferases.
- Uracil Phosphoribosyltransferase (UPRT): Uracil + PRPP ⇌ UMP + PPi
- Orotate Phosphoribosyltransferase (OPRT): Orotate + PRPP ⇌ OMP + PPi
PRPP is phosphoribosyl pyrophosphate and PPi is pyrophosphate.
4. Nucleotide Interconversion
Nucleotides can be interconverted to maintain the balance of different pyrimidine nucleotides:
- CTP Synthetase: UTP + Glutamine + ATP ⇌ CTP + Glutamate + ADP + Pi
- Ribonucleotide Reductase (RNR): UDP ⇌ dUDP CDP ⇌ dCDP
- Thymidylate Synthase (TS): dUMP + 5,10-Methylene-THF → dTMP + Dihydrofolate
Key Enzymes and Their Reactions
Here’s a comprehensive table of the key enzymes involved in the pyrimidine salvage pathway:
| Enzyme | Reaction | Function |
|---|---|---|
| Uridine-Cytidine Kinase (UCK) | Uridine/Cytidine + ATP ⇌ UMP/CMP + ADP | Phosphorylates uridine and cytidine |
| Thymidine Kinase (TK) | Thymidine + ATP ⇌ TMP + ADP | Phosphorylates thymidine |
| Deoxycytidine Kinase (dCK) | Deoxycytidine + ATP ⇌ dCMP + ADP | Phosphorylates deoxycytidine |
| Uracil Phosphoribosyltransferase (UPRT) | Uracil + PRPP ⇌ UMP + PPi | Converts uracil to UMP |
| Orotate Phosphoribosyltransferase (OPRT) | Orotate + PRPP ⇌ OMP + PPi | Converts orotate to OMP |
| CTP Synthetase | UTP + Glutamine + ATP ⇌ CTP + Glutamate + ADP + Pi | Converts UTP to CTP |
| Ribonucleotide Reductase (RNR) | UDP/CDP ⇌ dUDP/dCDP | Reduces ribonucleotides to deoxyribonucleotides |
| Thymidylate Synthase (TS) | dUMP + 5,10-Methylene-THF → dTMP + Dihydrofolate | Converts dUMP to dTMP |
Regulation of the Salvage Pathway
The pyrimidine salvage pathway is tightly regulated to maintain the proper balance of nucleotides. Key regulatory mechanisms include:
- Feedback Inhibition: End products inhibit earlier steps in the pathway. Example: CTP inhibits CTP synthetase
- Allosteric Regulation: Metabolites bind to enzymes and alter their activity. Example: ATP activates UMP kinase
- Transcriptional Regulation: Gene expression of salvage pathway enzymes is regulated. Example: E2F transcription factors regulate thymidine kinase expression
- Post-translational Modifications: Enzymes can be modified to alter their activity. Example: Phosphorylation of UMP kinase increases its activity
Comparison with De Novo Synthesis
While the salvage pathway is efficient, cells also rely on de novo synthesis. Here’s a comparison:
| Aspect | Salvage Pathway | De Novo Synthesis |
|---|---|---|
| Energy Cost | Lower | Higher |
| Precursors | Nucleosides and bases | Simple molecules (e.g., carbamoyl phosphate) |
| Regulation | Less complex | More complex |
| Speed | Faster | Slower |
| Importance in rapidly dividing cells | High | High |
Clinical Significance of the Pyrimidine Salvage Pathway
Understanding the pyrimidine salvage pathway has profound implications for clinical medicine, spanning drug development, genetic disorders, and cancer therapy. This section explores these areas in depth, highlighting the pathway’s importance in modern healthcare.
1. Drug Development
The pyrimidine salvage pathway serves as a crucial target for many antiviral and anticancer drugs. These medications, often nucleoside analogs, exploit the pathway’s mechanisms to exert their therapeutic effects.
1.1 Anticancer Drugs
Many anticancer drugs target the pyrimidine salvage pathway, interfering with DNA synthesis and cell proliferation.
Example: 5-Fluorouracil (5-FU)
5-Fluorouracil is a pyrimidine analog widely used in cancer treatment, particularly for colorectal and breast cancers.
Mechanism of action:
- 5-FU is converted to fluorodeoxyuridine monophosphate (FdUMP)
- FdUMP inhibits thymidylate synthase
- This inhibition leads to depletion of dTMP, disrupting DNA synthesis
- The result is cell cycle arrest and apoptosis in rapidly dividing cancer cells
Table: Comparison of 5-FU with natural pyrimidines
| Aspect | 5-Fluorouracil | Natural Uracil |
|---|---|---|
| Structure | Fluorine at C-5 position | Hydrogen at C-5 position |
| Metabolism | Converted to FdUMP | Converted to UMP |
| Effect on TS | Inhibits | Substrate |
| Cell cycle impact | Arrests S-phase | Normal progression |
1.2 Antiviral Drugs
Several antiviral drugs also target the pyrimidine salvage pathway, particularly those used against HIV, herpes viruses, and hepatitis B virus.
Example: Zidovudine (AZT)
Zidovudine, an antiretroviral medication, was the first approved treatment for HIV.
Mechanism of action:
- AZT is phosphorylated by cellular kinases
- The triphosphate form competes with natural dTTP
- When incorporated into viral DNA, it acts as a chain terminator
- This inhibits reverse transcriptase, preventing viral replication
2. Genetic Disorders
Defects in enzymes involved in the pyrimidine salvage pathway can lead to various genetic disorders, affecting nucleotide metabolism and cellular function.
Example: Thymidine Kinase 2 (TK2) Deficiency
TK2 deficiency causes mitochondrial DNA depletion syndrome, a severe condition affecting multiple organ systems.
Characteristics:
- Muscle weakness (myopathy)
- Neurological symptoms
- Impaired mitochondrial DNA maintenance
- Progressive course, often fatal in childhood
Table: Clinical Features of TK2 Deficiency
| System Affected | Symptoms |
|---|---|
| Muscular | Progressive weakness, hypotonia |
| Neurological | Encephalopathy, seizures |
| Respiratory | Respiratory failure |
| Gastrointestinal | Feeding difficulties, failure to thrive |
3. Cancer Therapy and Diagnostics
The unique characteristics of the pyrimidine salvage pathway in cancer cells provide opportunities for both targeted therapies and diagnostic techniques.
3.1 Targeted Therapies
Differences in nucleotide metabolism between normal and cancer cells can be exploited for selective treatment strategies.
Example: Capecitabine
Capecitabine is an oral prodrug that is preferentially activated in tumor cells.
Activation process:
- Capecitabine is absorbed intact in the intestine.
- It undergoes a three-step enzymatic conversion.
- The final step, catalyzed by thymidine phosphorylase, occurs preferentially in tumor tissues.
- This results in higher concentrations of the active drug in cancer cells.
3.2 Diagnostic Imaging
The salvage pathway’s activity can be used for diagnostic imaging, particularly in cancer detection and monitoring.
Example: 18F-FLT PET Imaging
18F-fluorothymidine (18F-FLT) is a radiopharmaceutical used in positron emission tomography (PET) imaging.
Mechanism and uses:
- 18F-FLT is a thymidine analog that is phosphorylated by thymidine kinase 1 (TK1).
- TK1 is upregulated in proliferating cells, especially cancer cells
- The trapped 18F-FLT-monophosphate accumulates in rapidly dividing tissues
- This allows visualization of tumor proliferation and assessment of treatment response
Table: Comparison of 18F-FLT with 18F-FDG in PET Imaging
| Aspect | 18F-FLT | 18F-FDG |
|---|---|---|
| Target | DNA synthesis (TK1 activity) | Glucose metabolism |
| Specificity for proliferation | Higher | Lower |
| Background in brain | Lower | Higher |
| Use in treatment response | Earlier changes are detectable | Standard of care |
4. Future Directions
Research into the clinical applications of the pyrimidine salvage pathway continues to evolve. Emerging areas include:
- Personalized Medicine: Tailoring treatments based on individual variations in salvage pathway enzymes
- Combination Therapies: Exploiting synergies between salvage pathway inhibitors and other anticancer agents
- Novel Imaging Techniques: Developing new tracers for more specific and sensitive diagnostic imaging
- Gene Therapy: Exploring potential treatments for genetic disorders affecting the salvage pathway
Frequently Asked Questions (FAQs)
Why is the salvage pathway important for cells?
The salvage pathway is important because it allows cells to recycle preformed nucleosides and bases, conserving energy and resources. It reduces the need for de novo synthesis and helps maintain the nucleotide pool.
What are the main differences between pyrimidines and purines?
Pyrimidines (cytosine, thymine, and uracil) have a single-ring structure, while purines (adenine, guanine) have a double-ring structure. Pyrimidines are generally smaller and have different metabolic pathways compared to purines.
How do antiviral drugs target the pyrimidine salvage pathway?
Many antiviral drugs are nucleoside analogs that can be phosphorylated by salvage pathway enzymes. Once phosphorylated, they can inhibit viral replication by interfering with viral DNA or RNA synthesis.
Can defects in the pyrimidine salvage pathway cause genetic disorders?
Yes, defects in enzymes involved in the pyrimidine salvage pathway can lead to various genetic disorders. For example, thymidine kinase 2 deficiency can cause mitochondrial DNA depletion syndrome.
How does the salvage pathway interact with the de novo synthesis pathway?
The salvage and de novo pathways are complementary. Cells can switch between these pathways based on their needs and the availability of precursors. The activity of one pathway can influence the other through various regulatory mechanisms to maintain nucleotide balance.
Conclusion
The salvage pathway of pyrimidine nucleotides is a complex and crucial aspect of cellular metabolism. Its intricate network of enzymes and reactions allows cells to efficiently recycle nucleosides and bases, maintaining the delicate balance of nucleotides necessary for DNA and RNA synthesis.
As research in this field continues to advance, our understanding of the salvage pathway will undoubtedly lead to new therapeutic strategies and biotechnological applications.