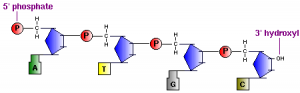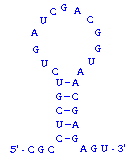
In Nucleic acid structures, there are two types of nucleic acid. They are DNA (deoxyribonucleic acid) and RNA (ribonucleic acid) are polymers of nucleotides linked in a chain through phosphodiester bonds.
In biological systems, they serve as information-carrying molecules or, in the case of some RNA molecules, catalysts. This brief review will focus on aspects of the structure of particular importance in manipulating DNA.

Basic compositions of Nucleic acid structures:
The basic structure of nucleic acids is Nitrogenous bases, the sugar moiety, and the Phosphate molecule.
- Nucleoside = Nitrogen base + Sugar
- Nucleotide = Nucleoside (Nitrogen base + Sugar) + Phosphate molecule
Nucleotides are the building blocks of all nucleic acids. Nucleotides have a distinctive structure composed of three components covalently bound together:
- Nitrogenous bases– Pyrimidine (one ring) and Purine (two rings)
- Sugar moiety – Ribose or Deoxyribose
- Phosphate molecule
The combination of a base and sugar is called a nucleoside. Nucleotides also exist inactivated forms containing two or three phosphates, called nucleotide diphosphates or triphosphates. If the sugar in a nucleotide is deoxyribose, the nucleotide is called a deoxynucleotide; if the sugar is ribose, the term ribonucleotide is used.
The structure of a nucleotide is depicted below. The structure on the left – deoxyguanosine – depicts the base, sugar, and phosphate moieties. In comparison, the structure on the right has an extra hydroxyl group on the 2′ carbon of ribose, making it a ribonucleotide – riboguanosine or just guanosine.
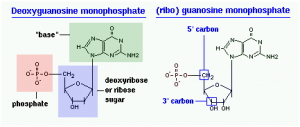
In the right-hand figure, note also the 5′ and 3′ carbons on ribose (or deoxyribose) – understanding this concept and nomenclature are critical to the understanding polarity of nucleic acids, as discussed below. The 5′ carbon has an attached phosphate group, while the 3′ carbon has a hydroxyl group.
There are five common bases, and four are generally represented in either DNA or RNA. Those bases and their corresponding nucleosides are described in the following table:
Another useful way to categorize nucleotide bases is as purines (A and G) versus pyrimidines (C, T, and U). Although committing this to memory is often difficult, the importance is that in double-stranded nucleic acids, base pairs are always formed between a purine and a pyrimidine.
Basic Nucleic acid structures are
Nucleic Acids:
DNA and RNA are synthesized in cells by DNA polymerases and RNA polymerases. Short fragments of nucleic acids also are commonly produced without enzymes by oligonucleotide synthesizers. In all cases, the process involves forming phosphodiester bonds between the 3′ carbon of one nucleotide and the 5′ carbon of another nucleotide. This leads to the formation of the backbone so-called “sugar-phosphate backbone”.
A key feature of all nucleic acids is that they have two distinctive ends: the 5′ (5-prime) and 3′ (3-prime) ends. This terminology refers to the 5′ and 3′ carbons on the sugar. For both DNA (shown above) and RNA, the 5′ end bears a phosphate, and the 3′ end a hydroxyl group.
Another important concept in the nucleic acid structure is that DNA and RNA polymerases add nucleotides to the 3′ end of the previously incorporated base. The nucleic acid synthesis direction is 5′ to 3′ direction (5’→3′).
Base Pairing and Double-Stranded Nucleic Acid structures:
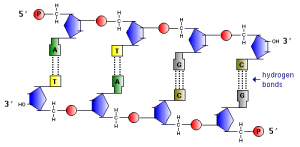
Most DNA exists in the famous form of a double helix, in which two linear strands of DNA are wound around one another. The major force promoting the formation of this helix is the complementary base pairing. Adenine forms hydrogen bonds with Thymine (or Uracils in RNA), and Guanine forms hydrogen bonds with Cytosine. If we mix two ATGC’s together, the following duplex will form:
Examine the figure above and note two very important features:
- The two strands of DNA are arranged antiparallel to one another: viewed from left to right the “top” strand is aligned 5′ to 3′, while the “bottom” strand is aligned 3′ to 5′. This is always the case for duplex nucleic acids.
- G-C base pairs have 3 hydrogen bonds, whereas A-T base pairs have 2 hydrogen bonds: one consequence of this disparity is that it takes more energy (e.g. a higher temperature) to disrupt GC-rich DNA than AT-rich DNA.
The above figures fail to impart an appreciation of the three-dimensional structure of DNA. This deficiency can be rectified to some extent by viewing and manipulating a 3-D model of duplex DNA.
What about double-stranded RNA? RNAs are usually single-stranded, but many RNA molecules have a secondary structure in which intramolecular loops are formed by complementary base pairing. A simple example of this is shown in the figure to the right, and much more extensive and complex examples are known. Base pairing in RNA follows exactly the same principles as with DNA: the two regions involved in duplex formation are antiparallel to one another, and the base pairs that form are A-U and G-C.
OK, what about RNA-DNA hybrids? Can they form? The answer is yes. Complementary sequences of RNA and DNA readily anneal with one another to form duplexes. In fact, RNA-DNA hybrids are more stable than the corresponding DNA-DNA and RNA-RNA duplexes.